Abstract
Introduction: Peripheral T-cell lymphomas (PTCL) are a heterogeneous group of lymphomas associated with poor outcomes following anthracycline-based chemotherapy, even when consolidative autologous stem cell transplantation (ASCT) is used. CD30 expression is universal in anaplastic large cell lymphoma (ALCL) and is frequently expressed in other PTCL subtypes. Brentuximab vedotin (BV) is a CD30-directed antibody drug conjugate that prolongs progression-free survival (PFS) and overall survival (OS) when combined with cyclophosphamide, doxorubicin, and prednisone (CHP) as compared to CHOP chemotherapy (Horwitz, 2020). Although a majority of pts treated with BV-CHP remained in durable remission (5y PFS 51%), there is room for improvement. Based on retrospective studies that demonstrated improved outcomes in younger pts, the addition of etoposide to CHOP (CHOEP) is commonly used as initial therapy for PTCL. We performed a multicenter phase 2 trial to evaluate the safety and efficacy of adding etoposide to BV-CHP (CHEP-BV) followed by BV consolidation in pts with newly diagnosed CD30-expressing PTCL.
Methods: Adults with newly diagnosed CD30+ (≥ 1% of tumor cells by local pathology) PTCL were eligible, including pts with ALK+ ALCL and IPI score ≥ 2, ALK-negative ALCL, PTCL not otherwise specified (NOS), angioimmunoblastic T-cell lymphoma (AITL), adult T-cell leukemia/lymphoma (ATLL), among others. After accrual of 28 pts, the protocol was amended to allow enrollment of 20 additional pts with CD30+ non-ALCL PTCL (with ALCL allowed in Canada). Pts could receive prephase steroids and/or 1 cycle of CHOP-equivalent chemotherapy prior to study entry. 6 pts were treated in a safety lead-in cohort and all pts received CHEP-BV at the recommended phase 2 dose: 6 x 21-day cycles of CHP+BV (1.8mg/kg) on d1 and etoposide 100mg/m2 on d1-3. G-CSF prophylaxis was mandatory. Pts in response after CHEP-BV could receive BV consolidation (1.8mg/kg q3w) for up to 10 additional cycles (16 total BV cycles) either after ASCT or CHEP-BV if no ASCT was performed. The co-primary endpoints were safety and the CR rate (Deauville score 1-3) by PET-CT after CHEP-BV assessed by investigators according to the 2014 Lugano classification. Secondary endpoints were PFS and OS.
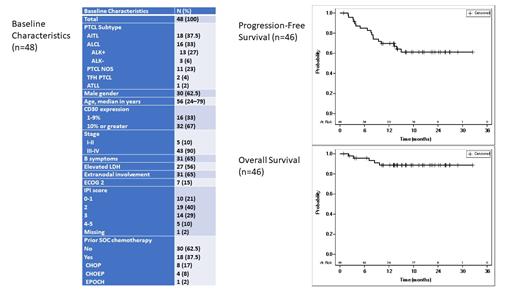
Results: Accrual has completed and 48 pts were enrolled; all were evaluable for toxicity, 46 were evaluable for efficacy. 16 pts had ALCL (13 ALK+, 3 ALK-) and 32 had non-ALCL PTCL subtypes, including 18 with AITL, 11 with PTCL NOS, 2 with T-follicular helper PTCL, and 1 with ATLL. Baseline characteristics are shown in Table. 43 pts completed CHEP-BV, 2 had progressive disease (PD) prior to completion, 1 pt discontinued CHEP-BV early (MD discretion), 1 pt died due to COVID-19, and 1 remains on CHEP-BV. Of 43 pts who completed CHEP-BV, 24 proceeded to ASCT and 19 did not. 33 (74%) pts received BV consolidation (20 after ASCT, 13 directly after CHEP-BV) and completed a median 8 of the planned 10 cycles (range, 1-10). 13 pts completed all cycles of consolidation; 19 pts discontinued early - 12 due to adverse events (AE), 5 due to PD, and 2 due to patient/physician choice. The most frequent CHEP-BV related AEs (all grades, G) include fatigue (73%), peripheral sensory neuropathy (67%), anemia (62.5%), nausea (56%), neutropenia (50%), lymphopenia (44%), leukopenia (42%), thrombocytopenia (40%), elevated transaminases (33%). The most common G3+ AEs were neutropenia (37.5%), febrile neutropenia (23%), lymphopenia (21%), anemia (19%), thrombocytopenia (19%). There were 5 deaths, 4 due to PD and 1 due to COVID-19 infection during C3 of CHEP-BV.
The interim (n=46) ORR and CR rates (after 3 CHEP-BV cycles, except 1 pt after 2) were 96% and 59% (27 CR, 17 PR), respectively. At completion of CHEP-BV (n=46), the ORR was 91% with 80% CR (37 CR, 5 PR, 4 PD). The ORR/CR rates in ALCL (n=16) vs non-ALCL (n=30) pts were 94%/94% vs 90%/73%, respectively. The ORR/CR rates in pts with CD30 expression 1-9% (n=15) vs 10+% (n=31) were 93%/67% and 90%/87%, respectively. The median follow-up in surviving pts is 16.1 months (range, 0.9-32.5). The overall 18mo PFS and OS were 61% and 89%; 18mo PFS by subgroup: ALCL 81%, non-ALCL 49%, CD30 1-9% 48%, CD30 10+% 67%. Landmark 1y PFS from end of CHEP-BV in responding pts (n=41) was 82% in pts who underwent ASCT vs 48% in pts who did not.
Conclusions: In a cohort of pts with mostly non-ALCL CD30-expressing PTCL, CHEP-BV (+/- ASCT) followed by BV consolidation was tolerable and effective.
Herrera: Genentech: Consultancy, Research Funding; Seagen: Consultancy, Research Funding; Kite, a Gilead Company: Research Funding; Gilead Sciences: Research Funding; Tubulis: Consultancy; Karyopharm: Consultancy; Bristol Myers Squibb: Consultancy, Research Funding; ADC Therapeutics: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Takeda: Consultancy; Merck: Consultancy, Research Funding. Zain: Secura Bio, DaichiSankyo, Abbvie: Research Funding; Kiyoaw Kirin, Secura Bio, Seattle Genetics: Honoraria; Secura Bio, Ono , Legend, Kiyowa Kirin, Myeloid Therapeutics Verastem Daichi Sankyo: Consultancy. Savage: Astra-Zeneca: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Takeda: Other: Institutional clinical trial funding; Roche: Research Funding; Servier: Consultancy, Honoraria; Merck: Consultancy, Honoraria, Other: Institutional clinical trial funding; AbbVie: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Other: Institutional clinical trial funding; Beigene: Other: Institutional clinical trial funding; Genentech: Research Funding. Feldman: Alexion, AstraZeneca Rare Disease: Honoraria, Other: Study investigator. Brammer: Celgene: Research Funding; Kymera Therapeutics: Consultancy; Seattle Genetics: Speakers Bureau. Popplewell: Hoffman La Roche: Other: Food; Novartis: Other: Travel; Pfizer: Other: Travel. Budde: Kite Pharma: Consultancy; Genentech: Consultancy, Research Funding; AstraZeneca: Research Funding. Mei: Morphosys: Research Funding; Janssen: Honoraria; TG Therapeutics: Research Funding; EUSA: Honoraria; BMS: Research Funding; Epizyme: Research Funding; Beigene: Research Funding. Leslie: Merck: Consultancy; Pharmacyclics: Consultancy, Honoraria, Speakers Bureau; Abbvie: Consultancy, Honoraria; PCYC/Janssen: Consultancy, Honoraria, Speakers Bureau; Kite, a Gilead Company: Consultancy, Honoraria, Speakers Bureau; Celgene/BMS: Consultancy, Honoraria, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; Karyopharm Therapeutics: Honoraria, Speakers Bureau; Epizyme: Consultancy, Honoraria, Speakers Bureau; Seagen: Consultancy, Honoraria, Speakers Bureau; BeiGene: Consultancy, Honoraria, Speakers Bureau; TG Therapeutics: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; ADC Therapeutics: Consultancy. Hosing: Nkarta Therapeutics: Membership on an entity's Board of Directors or advisory committees. Forman: Mustang Bio: Consultancy, Current holder of individual stocks in a privately-held company; Lixte Biotechnology: Consultancy, Current holder of individual stocks in a privately-held company; Allogene: Consultancy. Kwak: Pepromene Bio, Inc.: Consultancy, Current equity holder in publicly-traded company.